Cation Sample Prep and Analysis Protocol
Background
Major cations and trace metals are important constituents of sediment in many depositional settings. Different cations may be used for a range of paleo-analyses. For our purposes, trends in cation concentrations are first and foremost indicative of human pollution in the environment and associated land uses. Secondarily, cation fluctuations may indicate different sediment source areas with different geological settings.
Chemex Labs, Inc of Sparks, Nevada (775-356-5395) carries out determination of the chemical composition of our sediment samples. The procedure involves drying the samples and sieving them through a #80 mesh to obtain the fraction smaller than 0.177mm in diameter (Chemex code #201), treating them with hot concentrated nitric acid to destroy organic matter and oxidize sulfide material, and adding concentrated hydrochloric acid (3 times the volume of the nitric acid) to generate the aqua regia (3 HCl + HNO3 = 2 H2O + NOCl + Cl2) which is used to digest the material (Chemex code for the analysis is #229). This digestion is "total" for most base metals but is only "partial" for most major and minor elements. After digestion sample solutions are introduced into the core of an inductively-coupled argon plasma (ICP) at a temperature of approximately 8000 deg. C. At this temperature all elements become thermally excited and emit light at their characteristic wavelengths. This light is collected by the spectrometer and passes through a diffraction grating that serves to resolve the light into a spectrum of its constituent wavelengths. Within the spectrometer, this diffracted light is then collected by wavelength and amplified to yield an intensity measurement which can be converted to an elemental concentration by comparison with calibration standards. This measurement process is a form of atomic emission spectroscopy (AES), and the Chemex code is ICP-G32m (Fig. 1).
Since the analysis is performed on fine material, aluminum should be locked in the mineral structure and hence stable throughout the leaching process. Thus, by normalizing each element's concentration by that of aluminum, it is possible to correct for the different degrees of leaching in different samples. Ideally, a sediment standard from NIST with a known chemical composition should be sent with each batch for analysis to assess the degree of leaching independently.
Purpose
To determine the concentrations of cations in a sediment sample.
Materials
Mass balance with (0.001g resolution), sharpie, ziplock bags, plastic spatula.
Procedure
- Obtain a clean plastic tray and place on the mass balance; zero the balance to neglect for the mass of the tray.
- Homogenize the core sample by squeezing the zip-lock bag thoroughly.
- Using a plastic spatula, carefully remove sediment sample from the bag onto the plastic tray and attempt to weigh 50 g of material.
- Record the actual weight removed.
- Record the sample ID on a ziplock bag, along with the name of Gregory B. Pasternack, and the date.
- Remove the sample from the tray and place into bag.
- Rinse tray with DI water.
Data Analysis
- Create an Excel workbook with multiple worksheets.
- Put the raw data in the first worksheet with each ion in its own column.
- Paste the raw data into a 2nd sheet and organize the elements into the following groupings of columns:
- Major elements) Al, Fe, Ca, Mg, Na, K
- Secondary elements) Mn, Ti
- Nutrients) P, S
- Trace elements) Cu, Zn, Hg, Pb, As, Ba, Sr, Cd, Co, Cr, Ni, Ag
- Volcanic gases) B
- Others) Bi, Ga, La, Mo, Sb, Sc, Tl, U, V, W
- Raw concentrations of all elements deviate from true concentrations due to incomplete dissolution of sediment in the chemical procedure. Also, concentrations are strongly affected by sediment grain size, as clay and silt adsorb more metal ions than sand. To counteract these complications, you need to identify a major element that signifies the degree of leaching each sample experienced; usually that element is Al or Fe, but this must be assessed. To decide whether element concentrations are more positively correlated with Al or Fe, and thus which should be the "normalizing" element create a 3rd sheet. On it, plot Al vs Fe and Al versus Mg. Also, make a correlation matrix (R2 values) for the major elements using the function "=(CORREL(array1,array2))^2" and assess the degree of linear correlations among these elements. On the same sheet make a column of R2 values for Al versus each trace element and a column of R2 values for Fe versus each trace element.
- On a 4th sheet, create columns of "normalized" concentrations by dividing element concentrations by the corresponding value of the "normalizing" element. Plot and analyze the depth distributions of normalized concentrations to look for trends.
- The degree to which trace metals stem from anthropogenic sources as opposed to their natural association with inorganic sediment may be determined using a second standard geochemical normalization approach. To do this, a representative sample of unpolluted sediment from pre-Europeans settlement is needed. The basal sediments from a core (preferrably dated) may serve this purpose for each core. On a 5th sheet, divide the concentrations from each level in a core by the associated basal concentrations. The resulting values are the enrichment factors (EFs). EF-values greater than 1 indicate potential pollution anomolies, and there should be a distinctive increase above some date at which industry or land use began. Individual elements derive from either atmospheric or riverine sources depending on the local and regional history of industry and land use, which may be assessed through historical county records.
- If the core is not in an area where atmospheric or riverine pollution exists, then EF-values may vary widely through time above and below 1. In this case, EF-values are indicative of changing sediment sources. Ideally, surface samples from different soil types and geologic units should be used to try and identify the source regions.
- In order to compare cores against one another by chemical component, construct ternary diagrams that plot concentrations of three elements for all cores. Free ternary plotting programs such as Panplot can be downloaded off the web. Organize normalized core data by depth and construct separate columns for depths of 0-5 m, 5-10 m, and 10-o m. For each depth column, add the associated normalized chemical data. Each core should thus have three populations of chemical data differentiated only by range of depth. Plot the following ternary diagrams with the depth-structured, normalized chemical data using the PanPlot or similar program: Na:Ca:K, Al:Mg:Fe, Cu:Hg:Zn: Mn:P:Ti, and Ba:Pb:Ni.
Safety Issues
- Avoid using metal spatulas or any kind of metal during the analysis, as this can contaminate samples.
- When analyzing data, avoid eye strain by taking period breaks.
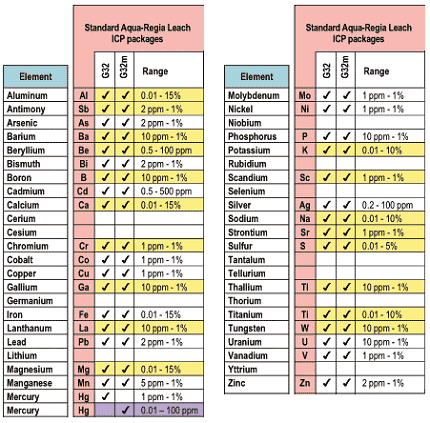
Figure 1. List of elements included in analysis. Yellow indicates those elements with only partial digestion. Purple indicates parts per billion resolution for Hg.


